41 fda approved drug labels
TWP's Guide to the FDA-approved Drug Label the fda-approved "drug label" is a document written by the manufacturer of a particular pharmaceutical drug in collaboration with the u.s. food and drug administration (fda), and is intended to provide patients and practitioners with key information about one or more approved uses of the drug in the united states, its main chemical properties, … Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs...
Drug Research and Children | FDA Before the Food and Drug Administration initiated a pediatric program, only about 20 percent of drugs approved by the FDA were labeled for pediatric use. By necessity, doctors have routinely given ...
Fda approved drug labels
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical devices. PDF Food and Drug Administration Food and Drug Administration FDA Label Search-Ingredient Name - Food and Drug Administration 10903 New Hampshire Avenue. Silver Spring, MD 20993. Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government. For Press.
Fda approved drug labels. FDA revises labels of SGLT2 inhibitors for diabetes to include … 15. maalisk. 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. Drug Approvals and Databases | FDA Drug and Biologic Approval and IND Activity Reports. Drug Trials Snapshots. Oncology (Cancer) / Hematologic Malignancies Approval Notifications. FDALabel. FDA Online Label Repository. FDA's ... FDALabel: Full-Text Search of Drug Product Labeling | FDA The following table lists the count of several common labeling types in FDALabel. * Includes Human OTC drugs approved for marketing through a New Drug Application (NDA), Abbreviated New...
COVID-19 Vaccines | FDA - U.S. Food and Drug Administration The FDA approved a manufacturing change for Comirnaty to include a formulation that uses a different buffer; and an abbreviated new drug application for increase blood pressure in adults with ... PDF Reference ID: 3397413 - Food and Drug Administration antipsychotic drugs are at an increased risk of death. SEROQUEL is not approved for elderly patients with dementia-related psychosis (5.1) Suicidal Thoughts and Behaviors • Increased risk of suicidal thoughts and behavior in children, adolescents and young adults taking antidepressants (5.2) • Drugs and Biologicals, Coverage of, for Label and Off-Label Uses Coverage Indications, Limitations, and/or Medical Necessity. Abstract: An off-label/unlabeled use of a drug is defined as a use for a non-FDA approved indication, that is, one that is not listed on the drug's official label/prescribing information. An indication is defined as a diagnosis, illness, injury, syndrome, condition, or other clinical ... FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing...
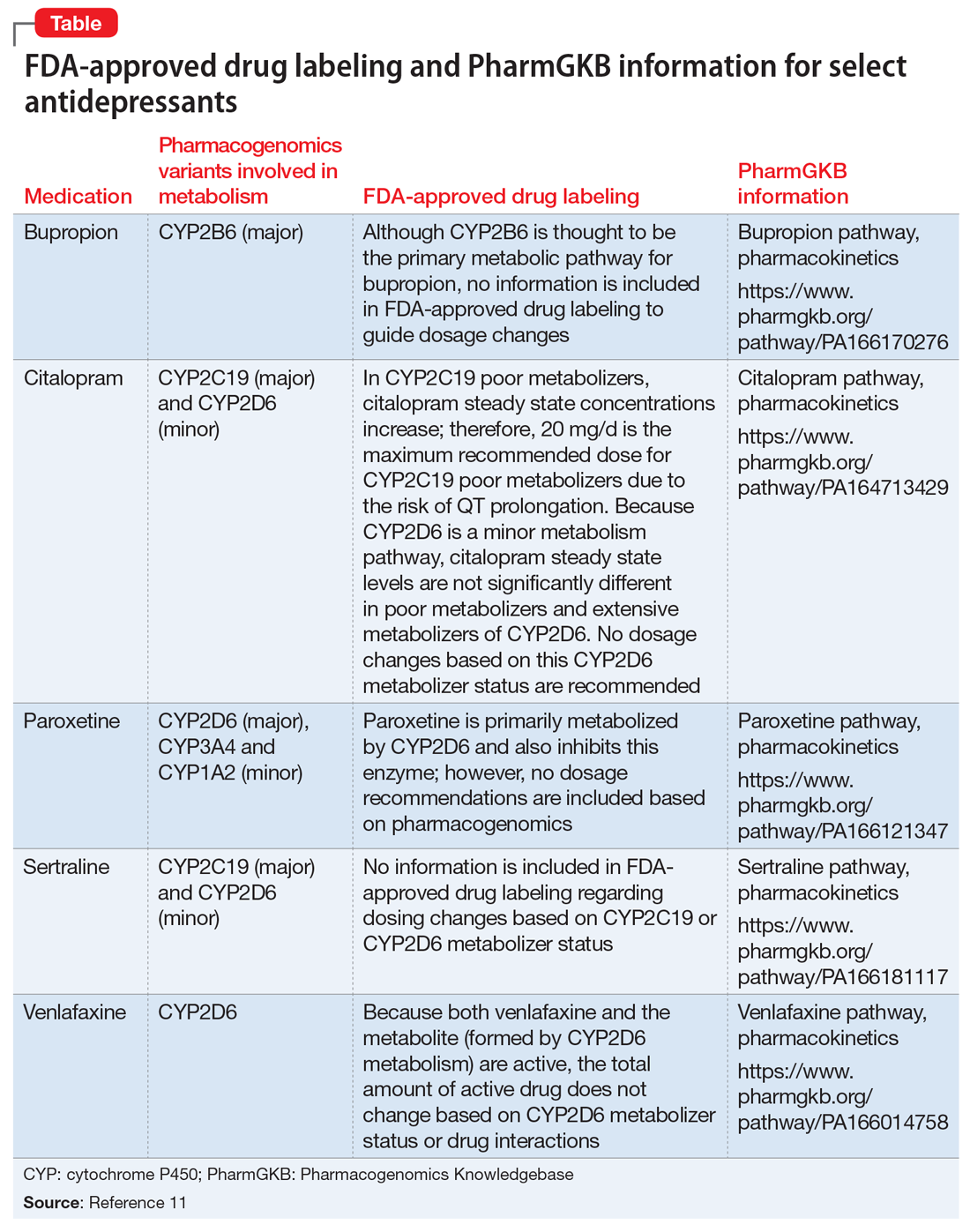
PDF Celexa® (citalopram hydrobromide) Tablets - Food and Drug Administration Drug-Drug Interactions . In vitro enzyme inhibition data did not reveal an inhibitory effect of citalopram on CYP3A4, 2C9, or -2E1, but did suggest that it is a weak inhibitor of CYP1A2, -2D6, and -2C19. Citalopram would be expected to have little inhibitory effect on in vivo metabolism mediated by these enzymes. FDA Drug Safety Communication: FDA warns about new impulse … [05-03-2016] The U.S. Food and Drug Administration (FDA) is warning that compulsive or uncontrollable urges to gamble, binge eat, shop, and have sex have been reported with the use of the ... FDA Drug Safety Communication: FDA updates warnings for oral … [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). Pet Food Labels - General | FDA - U.S. Food and Drug Administration Other possible ingredients may include artificial colors, stabilizers, and preservatives. All should be either "Generally Recognized As Safe (GRAS)" or approved food additives for their intended uses.
FDA Drug Safety Communication: FDA cautions about using … [03-03-2015] The U.S. Food and Drug Administration (FDA) cautions that prescription testosterone products are approved only for men who have low testosterone levels caused by certain medical ...
FDA Label Search - Food and Drug Administration The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and...
FDA Antidepressant Drug Labels for Pregnant and Postpartum Women FDA Antidepressant Drug Labels for Pregnant and Postpartum Women - Screening for Depression in Adults - NCBI Bookshelf On October 7, 2014, we searched for the current drug label information of brand name antidepressants on the Drugs@FDA website ( ).
Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
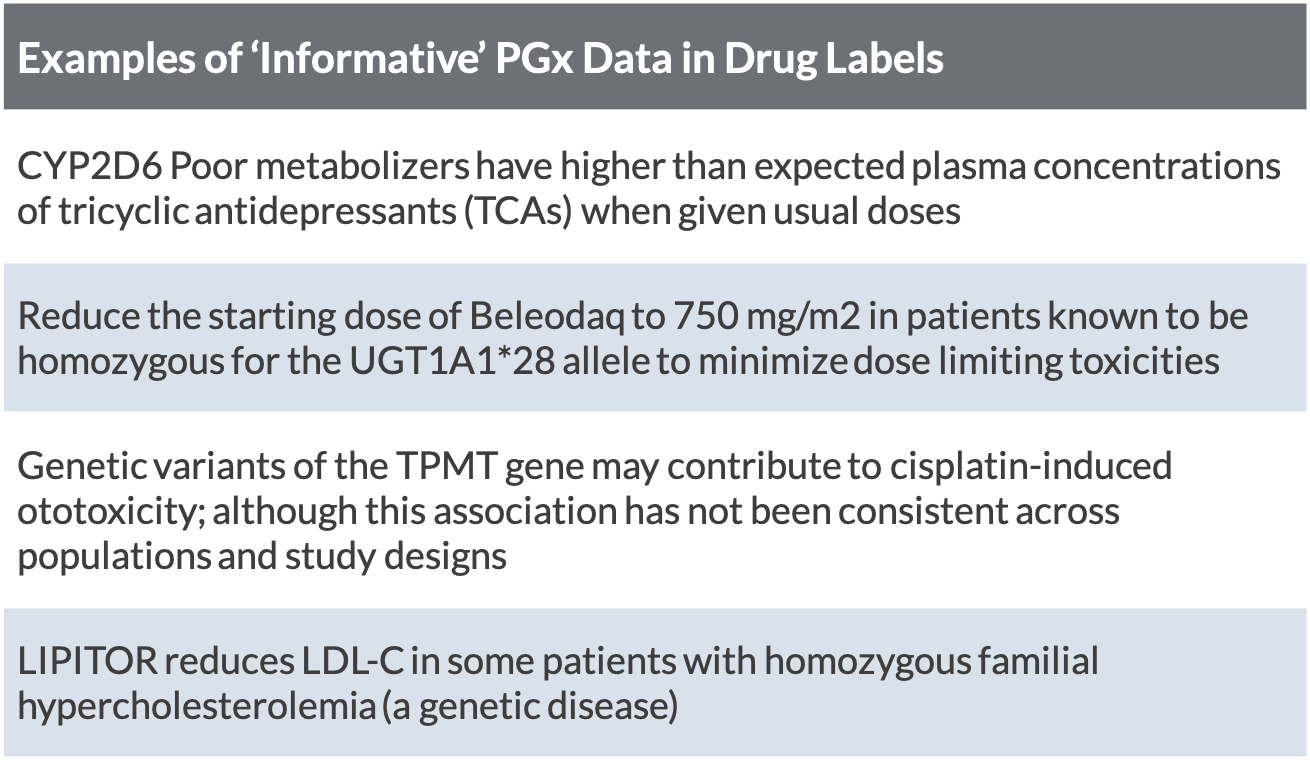
Pharmacogenetic Labeling of FDA-Approved Drugs - PMC The U.S. Food and Drug Administration recently marked 10 years since first updating the labeling for warfarin (often referred to as the "poster child" of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks.
Drug Safety-related Labeling Changes (SrLC) Database Drug Safety-related Labeling Changes (SrLC) Database Overview: Updates to Safety Information in FDA-Approved Prescription Drug Labeling Contact FDA Toll Free (855) 543-3784, or (301)...
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling...
PDF label - Food and Drug Administration Initial U.S. Approval: 2004 -----RECENT MAJOR CHANGES----- Indications and Usage (1) 6/2012 Dosage and Administration (2.5) 6/2012 ... See 17 for PATIENT COUNSELING INFORMATION and FDA-approved Medication Guide Revised: 6/2012 . Reference ID: 3148643 ... Withdrawal of Antiepileptic Drugs (AEDs) 5.4. Suicidal Behavior and Ideation 5.5 ...
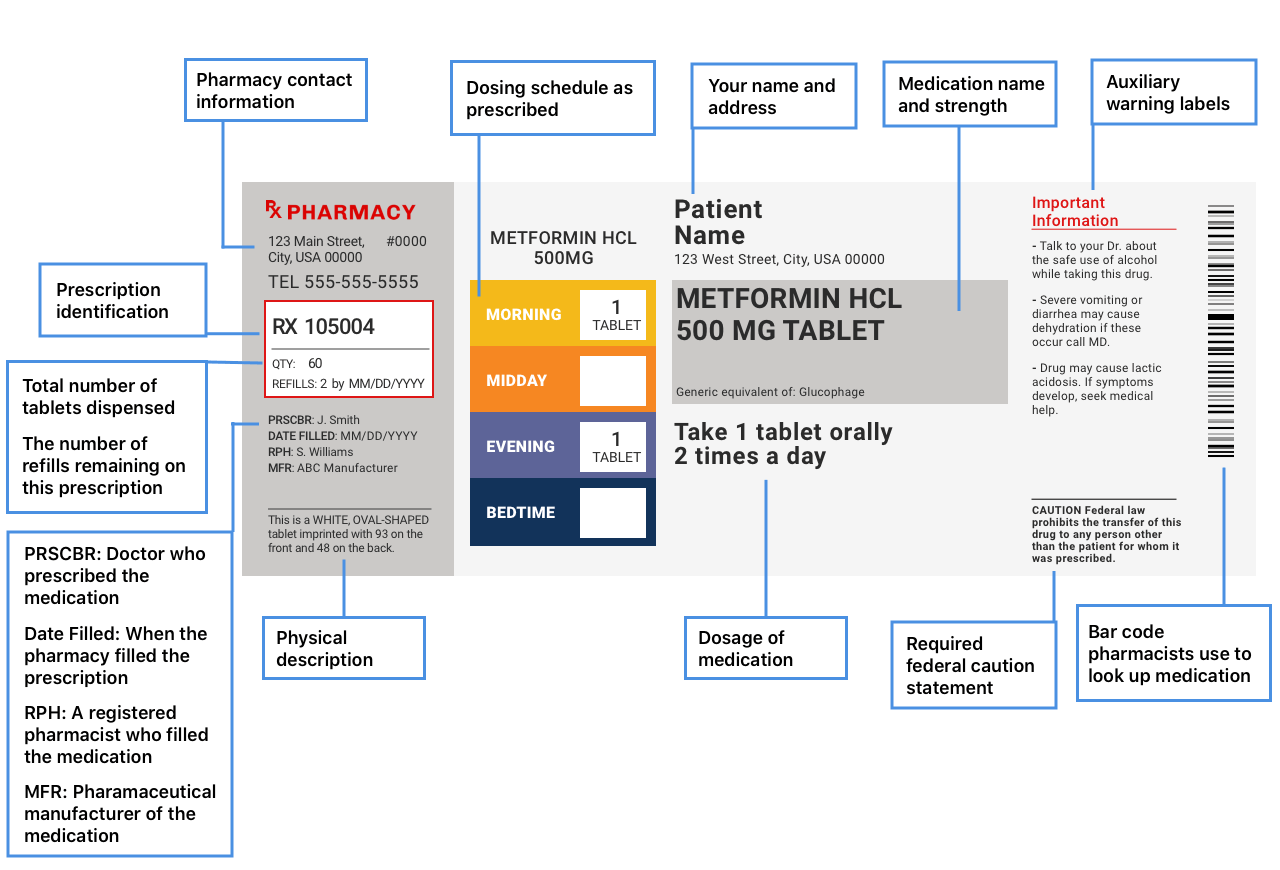
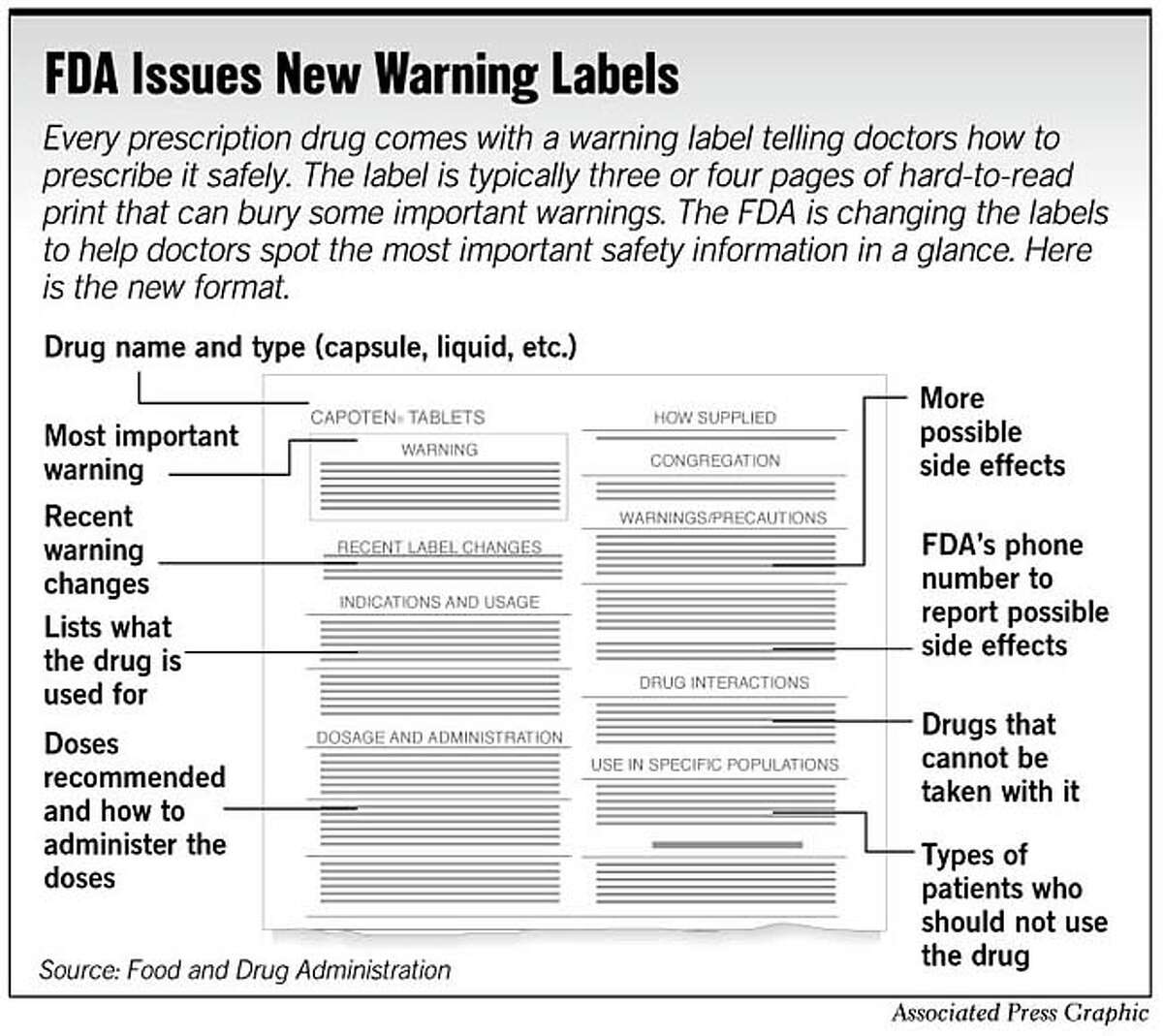
Pharmaceutical Labeling 101: FDA Regulations Guide The FDA has a strict code when it comes to medicine labeling and all manufacturers, big or small, must adhere to these rules to get their product FDA approved. All human prescription drugs and biological products should follow the guidelines available in 21 CFR 201.56(d) and 201.57.
Clinical pharmacology information in regulatory submissions and ... Overall, although there was a trend for more labeling recommendations and fewer postmarketing studies and clinical trials for non-orphan drugs, there appeared to be no substantial differences in how these select clinical pharmacology studies are leveraged during the development and approval of orphan and non-orphan drugs.
Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. [3] An example of FDA-approved labeling is the Professional Package Insert (PPI).
FDA Label Search-Package Code - Food and Drug Administration FDA Label Search. FDA Home - Search by NDC: (Type the 4 or 5 digit NDC Labeler Code with the hyphen (e.g., 0001-), the 8 or 9 digit NDC Product Code (e.g., 0001-0001) or the 10 digit NDC (0001-0001-01)) ... U.S. Food and Drug Administration. 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For ...
FDALabel - U.S. Food and Drug Administration Labeling, Product and Ingredient Identifiers. Application Number for ANDA, BLA, or NDA: 3 to 6 digits (e.g., 077844, 125118, 020977) Unique Ingredient Identifier (UNII): To search for active ingredients, inactive ingredients or both, type in alphanumeric code (s) (e.g., J220T4J9Q2)
PDF TOPAMAX (topiramate) Label - Food and Drug Administration Suicidal behavior and ideation: antiepileptic drugs increase the risk of suicidal behavior or ideation (5.5) Cognitive/neuropsychiatric adverse reactions: use caution when operating machinery including cars; depression and mood problems may occur (5.6) Fetal Toxicity: use during pregnancy can cause cleft lip and/or palate and
Approved Animal Drug Products (Green Book) | FDA 14. helmik. 2022 · Most FDA-approved animal drugs are included in a publicly available list of approved animal drug products. This list is called the Green Book for short, and FDA updates it in its entirety every month.
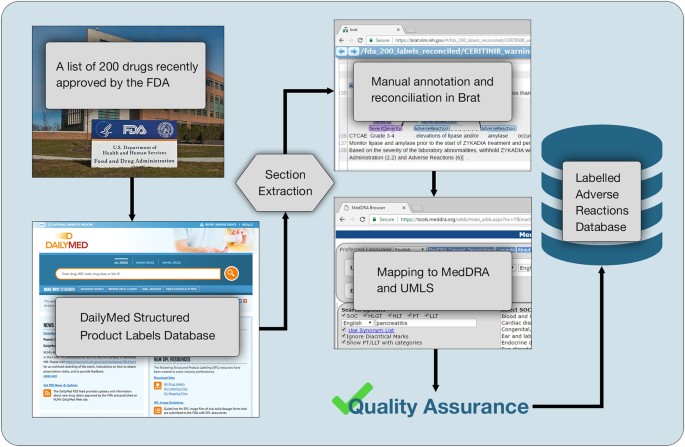
Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for...
Is It Really 'FDA Approved'? - U.S. Food and Drug Administration 10. toukok. 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA 11. elok. 2022 · Pharmacogenomics can play an important role in identifying responders and non-responders to medications, avoiding adverse events, and optimizing drug dose. Drug labeling may contain information on ...
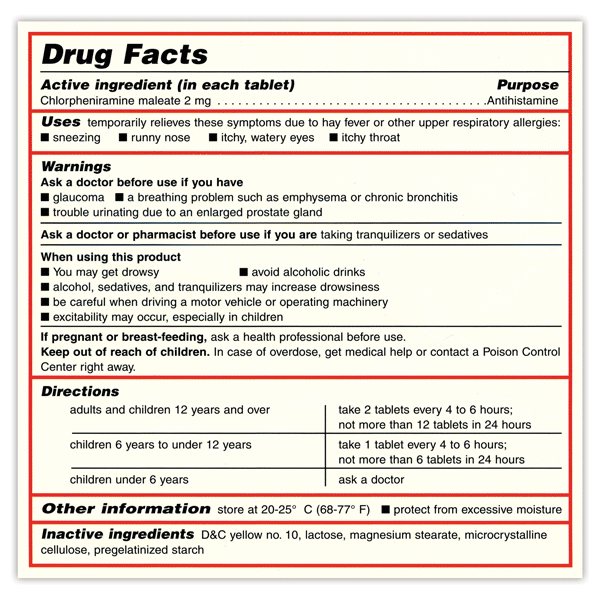
OTC Drug Facts Label | FDA In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new...
Coronavirus (COVID-19) Update: FDA Authorizes Moderna and … Jun 17, 2022 · The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines ...
Recommend one FDA-approved drug, one off-label drug, and one... Part 1. There really are FDA-approved medications, off-label pharmaceuticals, and non-pharmacological therapies for treating PTSD in adolescents. FDA-Approved Drugs: The FDA has authorized zoloft (Zoloft) and amitriptyline for the therapy of PTSD in kids and adolescents (Paxil). Both of these medications are antidepressants known as SSRIs.
FDA Label Search-Ingredient Name - Food and Drug Administration 10903 New Hampshire Avenue. Silver Spring, MD 20993. Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government. For Press.
PDF Food and Drug Administration Food and Drug Administration
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical devices.




















.jpg)






Post a Comment for "41 fda approved drug labels"