38 health claims food labels
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims) Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient...
Health Claims - Canada.ca Health Claims Health Canada recognizes that the foods we eat can affect our health in different ways. Some food labels contain statements about the beneficial effects of certain foods on a person's health, such as " a healthy diet low in saturated and trans fat may reduce the risk of heart disease ".
Health claims food labels
Qualified Health Claims | FDA - U.S. Food and Drug Administration For a QHC petition with credible scientific evidence, the FDA issues a Letter of Enforcement Discretion including specific claim language that reflects the level of supporting scientific evidence... Health Claims on Food Labels | Kaiser Permanente Health claims may be statements like: "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis." "Development of cancer depends on many factors. A diet low in total fat may reduce the risk of some cancers." But just because a food label has a health claim does not mean that the food is healthy for ... Health Claims on Food Labels - LabelCalc A lot of new food manufacturers ask me about making health claims on food labels, so today I wanted to shed some light on what can sometimes be a complex process. In order to successfully navigate the world of claims for your food product—from health claims to nutrient content claims and everything in between—here are the FDA rules you need ...
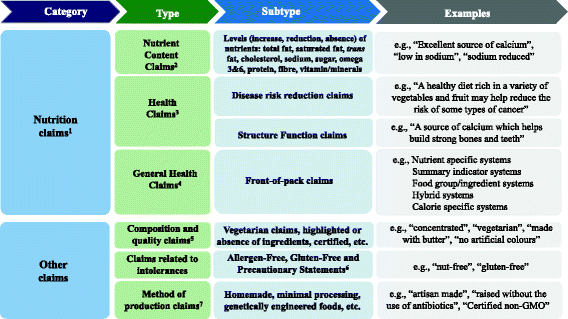
Health claims food labels. In Pictures: 29 Foods With "Health Claims" That ... - Modern Health Monk #6 Chocolate Milk - "Vitamins A&D 37% Less Fat Than Whole Milk!" Ingredients? Okay, so there's actually milk in this. Good sign. Ingredient #2 = sugar. Ingredient #3-6 = cocoa mix science experiment & preservatives. Ingredient # 4 = Artificial flavors. De-lish. #7 Arizona Iced Tea "NO Calories!" At first we're like, "oh, sweet, no calories!?" Everything you need to know about Health Claims on Food Labels A nutrient content claim must be true and accurate just like health claims, it is a statement about the amount of a nutrient found in a food. Usually placed on the front of the food label, the nutrient claim provides a quick comparison between similar products. Some examples of nutrient claims are "low sodium", "high in fiber", " fewer calories", 23 Misleading Food Label Claims (+What They Really Mean!) - SkinnyFit 2. Nutrient Content Claims. A nutrient content claim is a guide to help you consume more or less of a certain nutrient. They must be true and accurate just like health claims. An example of a nutrient content claim is a food label that says "low in fat" or "good source of calcium". 3. Structure/Function Claims. Label Claims for Conventional Foods and Dietary Supplements the nutrition labeling and education act of 1990 (nlea) provides for the use in food labeling of health claims that characterize a relationship between a food, a food component, or...
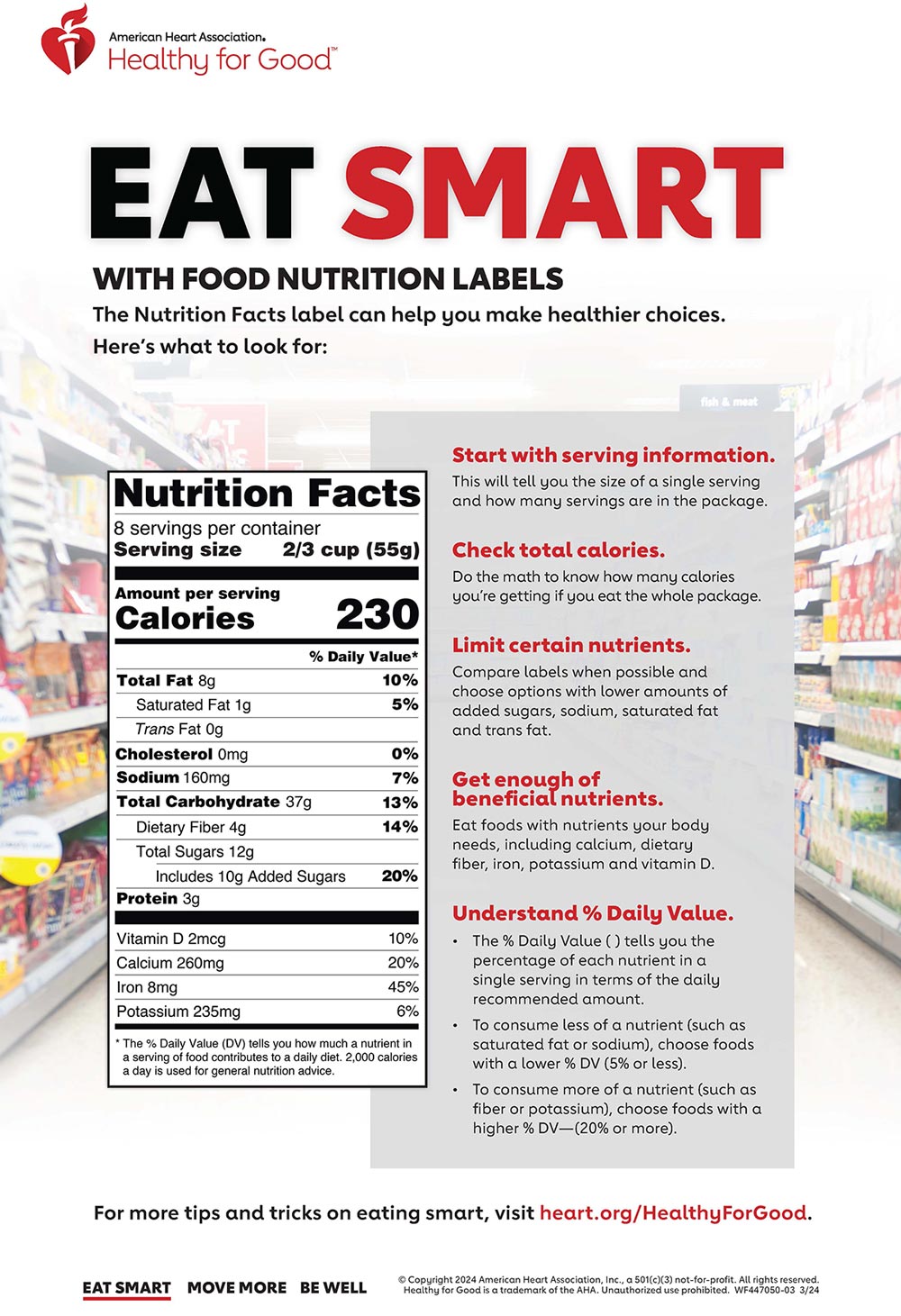
Health claims on food labels - PubMed Health claims on food labels Mil Med. 1994 Mar;159(3):213-7. Author L Tollefson 1 Affiliation 1 Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC 20204. PMID: 8041466 Abstract Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no ... Authorized Health Claims That Meet Significant Scientific Agreement Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products or dietary supplements to show that a food or food component may... Nutrient Claims on Food Labels - Clemson University Sodium content cannot exceed 360 mg per serving for individual foods and 480 mg per serving for meal-type products. If a food is labeled "healthy" or makes a health claim, it cannot contain any nutrient that increases the risk for disease. It must contain no more than 20% of the DV per serving of total fat, saturated fat, cholesterol, or sodium. Marketplace tests 5 popular foods with healthy-sounding claims that may ... CBC Marketplace takes a closer look at five popular foods whose labels make healthy claims that may be too good to be true. Vexed by Vector The most misleading, say these nutrition experts, is...
Questions and Answers on Health Claims in Food Labeling Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a... Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. Health Claims on Food Labels - LabelCalc A lot of new food manufacturers ask me about making health claims on food labels, so today I wanted to shed some light on what can sometimes be a complex process. In order to successfully navigate the world of claims for your food product—from health claims to nutrient content claims and everything in between—here are the FDA rules you need ... Health Claims on Food Labels | Kaiser Permanente Health claims may be statements like: "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis." "Development of cancer depends on many factors. A diet low in total fat may reduce the risk of some cancers." But just because a food label has a health claim does not mean that the food is healthy for ...
Qualified Health Claims | FDA - U.S. Food and Drug Administration For a QHC petition with credible scientific evidence, the FDA issues a Letter of Enforcement Discretion including specific claim language that reflects the level of supporting scientific evidence...


















:max_bytes(150000):strip_icc()/juicy-juice-no-sugar-400x400-b1fb04c46e9e4c8392ce8881614c021a.jpg)













Post a Comment for "38 health claims food labels"